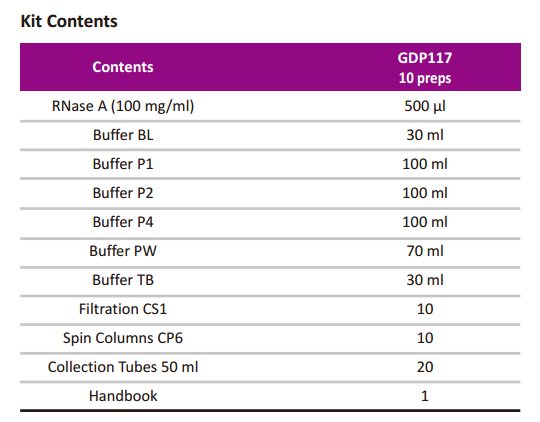
GDP117-EndoFree Maxi Plasmid Kit
EndoFree Maxi Plasmid Kit——For purification of ultrapure plasmid DNA with high yield from 100-200 ml
overnight bacteria culture
EndoFree Maxi Plasmid Kit
(Spin Column)
Cat.no. GDP117
Storage
EndoFree Maxi Plasmid Kit can be stored dry at room temperature (15-30°C) for up to 15 months without showing any reduction in performance and quality. If any precipitate forms in the buffers, it should be dissolved by warming the buffers to 37°C before use. RNase A (100 mg/ml) can be
stored for 15 months at room temperature (15-30°C). After adding RNase A,
Buffer P1 is stable for 6 months at 2-8°C.
Introduction
EndoFree Maxi Plasmid Kit is based on alkaline lysis technology followed
by adsorption of DNA onto silica membrane in the presence of high salt,
and uses Buffer P4 and Filtration CS1 to wipe off the endotoxin and protein
impurity effectively. Plasmid DNA prepared by EndoFree Plasmid Kit is
suitable for a variety of routine applications including restriction enzyme
digestion, sequencing, library screening, ligation and transformation, in
vitro translation, and transfection of robust cells.
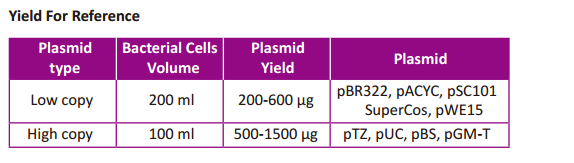
Recommended bacterial culture volume: 500-1500 μg plasmid with 100
ml bacterial culture for high-copy vectors; and 200-600 μg plasmid with
200 ml bacterial culture for low-copy vectors.
Important Notes Please read before use.
- Add the provided RNase A solution to Buffer P1(use 1 vial RNase A per
bottle Buffer P1), mix, and store at 2-8°C. - Check Buffer BL, P2 and P4 before use for salt precipitation. If necessary,
dissolve the buffer by warming at 37°C. - Avoid direct contact of Buffer P2 and P4, immediately close the lid after
use. - Draw out the plunger from the Filtration CS1 slowly to avoid membrane
loose. - The amount of extracted plasmid is related to cells concentration and
plasmid copy. If working with low copy vectors or large plasmid (>10 kb),
it may be beneficial to increase culture volume and to increase Buffer
P1, P2, and P4 in proportion. Warm the Buffer TB to 65-70°C before use.
Prolong adsorption and elution time properly to increase extraction
efficiency. - Use Buffer BL to treat spin columns could activate silica membrane at
maximum degree and higher yield. - After treated with Buffer BL, use the spin columns soon, since long-term
placement may affect the purification effect.
Reagents need to be prepared by Customer96-100% ethanol, isopropanol, 5M NaCl (optional) and 70% ethanol (optional).
Protocol
Add ethanol (96-100%) to Buffer PW before use, check bottle tag for the
adding volume.
- Column equilibration: place a Spin Column CP6 into a 50 ml Collection
Tube (supplied in the kit) and add 2.5 ml Buffer BL to Spin Column
CP6. Centrifuge for 2 min at 8,000 rpm (~8,228 × g). Discard the flowthrough, and place Spin Column CP6 into the same Collection Tube (use
the spin columns as soon as possible after treated with Buffer BL). - Harvest 100 ml (for low copy plasmid, please harvest 200 ml) overnight
cultured bacterial cells by centrifuging at 8,000 rpm (~8,228 × g) for
3 min at room temperature (15-30°C), and then remove all traces of
supernatant.
Note: For large volume of bacterial cells, please harvest to one tube
by several centrifugation steps. Too much bacterial cells will lead to
incomplete lysis and further reduce plasmid yield. - Try to remove all traces of supernatant, use clean paper tissue to absorb
the fluids inside the tube wall. - Resuspend pelleted bacterial cells in 8 ml Buffer P1 (Ensure that RNase
A has been added). The bacteria should be resuspended completely by
vortex or pipetting up and down until no cell clumps remain.
Note: No cell clumps should be visible after re-suspension of the
pellet, otherwise incomplete lysis will lead to lower yield and purity.
For low copy plasmid, please increase Buffer P1, P2 and P4 volume too
when increase bacterial cell volume. - Add 8 ml Buffer P2 and mix thoroughly by inverting the tube 6-8 times,
then incubate at room temperature for 5 min.
Note: Mix by inverting the tube. Do not vortex, as this will result in
shearing of genomic DNA. If necessary, continue inverting the tube until the solution becomes viscous and clear. If the solution won’t turn clear, please reduce the amount of cells. - Add 8 ml Buffer P4, and mix immediately and thoroughly by gently
inverting 6-8 times, until the whole solution become cloudy. Incubate
at room temperature for 10 min. Centrifuge for 5-10 min at 8,000
rpm (~8,228 × g), the white material should be in the bottom of the
centrifuge tube(prolong centrifugation time properly). Transfer the
supernatant into a Filtration CS1 (avoid transferring large clump into the
Filtration CS1, which will clog the filtration membrane). Gently insert
the plunger into the Filtration CS1 and filter the cell lysate into a new 50
ml tube (not supplied in the kit).
Note: To avoid localized precipitation, mix the lysate thoroughly and
immediately after addition of Buffer P4. It will not affect filtration if
there is small white precipitate in the supernatant that transferred
to Filtration CS1. If using more than 100 ml bacterial culture, prolong
centrifugal time to 20-30 min. - Add 0.3×volume isopropanol to the cleared lysate (too much
isopropanol will lead to RNA contamination), mix completely by
reverting upside and down and then transfer all solution to the Spin
Column CP6 (put Spin Column CP6 into 50 ml Collection Tube).
Note: The filtrate volume will loss after filtration, so added volume
of isopropanol should be determined according to real filtrate
volume. It is recommended to apply no more than 10 ml of filtrate to
Spin Column CP6. For larger volumes, please divide the filtrate and
centrifuge for several times. - Centrifuge for 2 min at 8,000 rpm (~8,228 × g). Discard the flow-through
and place the Spin Column CP6 back into the same Collection Tube.
Note: Centrifuge for two times under the above condition. - Add 10 ml Buffer PW (Ensure ethanol has been added before use)to
the Spin Column CP6 and centrifuge at 8,000 rpm (~8,228 × g) for 2 min.
Discard the flow-through and place the Spin Column CP6 back into the
same Collection Tube. - Repeat step 9.
- Add 3 ml 100% ethanol to the Spin Column CP6 (put the CP6 in a Collection Tube). Centrifuge for 2 min at 8,000 rpm (~8,228 × g),discard the flow-through.
- Put Spin Column CP6 back to Collection Tube, centrifuge at 8,000 rpm (~8,228
× g) for 5 min for removing residual ethanol.
Note: We suggest opening Spin Column CP6 lid and stay at room temperature
for a while to completely dry the membrane. - To elute DNA, place the Spin Column CP6 in a clean 50 ml Collection Tube
(supplied in the kit) and add 1-2 ml Buffer TB to the center of the membrane
and incubate 5 min at room temperature, centrifuge at 8,000 rpm (~8,228 ×
g) for 2 min. Transfer the eluate from 50 ml centrifuge tube to a clean 1.5 ml
centrifuge tube and put at -20 °C for storage.
Note: Repeat step 13 to increase plasmid recovery efficiency. If the volume of
elution buffer is less than 1 ml, it may affect recovery efficiency. DNA product
should be stored at -20°C to avoid degradation. The pH value of elution
buffer will have big influence in eluting. If using distilled water, pH should be
controlled at 7.0-8.5, below 7.0 will affect elution efficiency.
An optional step
If you want to elevate the concentration of the plasmid, please finish the optional
step.
- Add 1.42 ml isopropanol and 0.42 ml 5M NaCl (prepared by the customer) to 1
ml plasmid elution buffer and mix completely. Incubate at room temperature
for 5 min. Centrifuge for 10 min at 8,000 rpm (~8,228 × g), and discard the
supernatant. - Add 0.5 ml 70% ethanol and Centrifuge for 10 min at 8,000 rpm (~8,228 × g),
and then remove residual ethanol. - Repeat step 15.
- Air-dry the washed plasmid at room temperature for 5-10 min, and add mall
volume TB buffer to dissolve the plasmid.
GDP117-EndoFree Maxi Plasmid Kit
All trademarks or registered trademarks appearing on this website are the property of their respective owners.
This product is for scientific research use only. Do not use in medicine, clinical treatment, food or cosmetics.
Need more info ? Contact us anytime. We’re here: Go2biotech
E-mail: maggie@go2biotech.com / morgan@go2biotech.com
Telephone:+86 755 8399 5017


