DP423-DNA/RNA/Protein Isolation Kit
DNA/RNA/Protein Isolation Kit
For simultaneous purification of genomic DNA, total RNA, and total protein from
the same cell or tissue sample
DNA/RNA/Protein Isolation Kit(Spin Column)
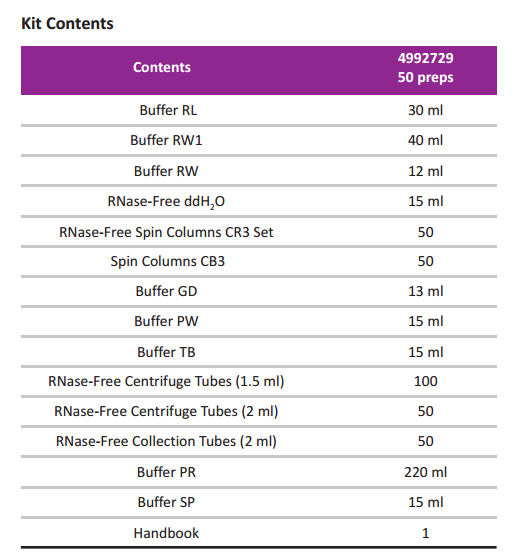
Cat. no. 4992729
Compatible Reagents
DNase I (Cat. no. 4992232)
Storage
Buffer RL added with β-mercaptoethanol could be stored for 30 days at
2-8°C. All the other reagents should be stored dry at room temperature
(15-30°C) and is stable for 15 months.
Introduction
This Kit is designed to purify genomic DNA, total RNA, and total protein
simultaneously from a single biological sample (cultured cells and animal
tissues) and allows the parallel processing of multiple samples.
RNA Protection
- Wear gloves when handling RNA and all reagents, as skin is a common
source of RNase. Change gloves frequently. - Use RNase-Free certified, disposable plastic ware and filter tips whenever possible.
- Buffer RL could protect RNA. But for experiment, RNA should be stored
or applied in RNase-Free plastic or glassware. To inactivate RNase, the
glassware could be dried at 150°C for 4 hours, while plastics could be
dipped in 0.5 M NaOH for 10 min, and washed by RNA-Free ddH2O
thoroughly and sterilized. - Use RNase-Free ddH2O to prepare solution (RNase-Free ddH2O: add
0.1 ml DEPC to 100 ml ddH2O and shake vigorously to bring DEPC into
solution. Let the solution stand overnight. Autoclave to remove any
trace of DEPC).
Important Notes Before Starting
- Add β-mercaptoethanol (β-ME) to Buffer RL to a final concentration
of 1% before use. For example, add 10 μl β-mercaptoethanol (β-ME)
per 1 ml Buffer RL. Buffer RL may form precipitate during storage. If
necessary, redissolve by warming it at 56°C, and then equilibrate to
room temperature. - Before use, add ethanol (96-100%) to Buffer RW, Buffer PW and Buffer
GD for the working solution, as described on the tag. - Perform all steps of the procedure at room temperature (15-30°C) if not
emphasized. - For some sensitive RNA samples, genomic DNA may need to be
removed completely in the following application. Please refer the oncolumn DNase I digestion procedure.
Protocol
Simultaneous Purification of Genomic DNA and Total RNA from Cultured
Cells
- Cell harvest :
Cells grown in suspension (do not use more than 1× 10
7
cells):
Determine the number of cells. Pellet the appropriate number of cells
by centrifuging for 5 min at 300 × g in a centrifuge tube. Carefully remove
all supernatant by aspiration, and proceed to step2.
Cells grown in a monolayer (do not use more than 1× 10
7
cells):
Cells grown in a monolayer in cell-culture vessels could be either lysed
directly in the vessel (up to 10 cm diameter) or trypsinized and collected
as a cell pellet prior to lysis. Cells grown in a monolayer in cell-culture
flasks should always be trypsinized.
1) To lyse cells directly: Determine the number of cells. Completely
aspirate the cell-culture medium, and proceed immediately to step2.
2)To trypsinize and collect cells: Determine the number of cells. Aspirate
the medium, and wash the cells with PBS. Aspirate the PBS, and add
0.10-0.25% trypsin into PBS. After the cells detach from the dish or
flask, add medium containing serum to inactivate the trypsin, then
transfer the cells to an RNase-Free glass or polypropylene centrifuge
tube (not supplied), and centrifuge for 5 min at 300 × g. Completely
aspirate the supernatant, and proceed to step2.
Note: Incomplete removal of cell-culture medium will inhibit lysis
and dilute the lysate, affecting the conditions for nucleic acid
purification. Both effects may reduce nucleic acid yields and purity.
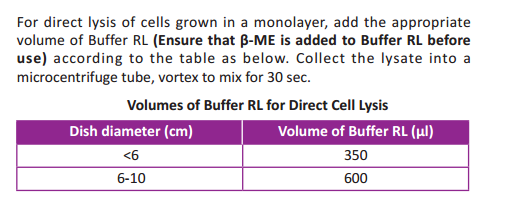
- Lyse the cells by adding Buffer RL:
For pelleted cells, loosen the cell pellet thoroughly by flicking the tube.
Add the appropriate volume of Buffer RL according to the following
table, vortex for 30 sec.(Ensure that β-ME is added to Buffer RL before
use).

- Pipet the lysate directly into a Spin Column CB3 placed in a 2ml
collection tube, and centrifuge for 30-60 sec at 12,000 rpm (~13,400 × g)
to collect the filtrate. Place the Spin Column CB3 into a collection tube
at room temperature or 4°C for later DNA purification.
DNase I digestion procedure (optional)
Preparation of DNase I stock solution: Dissolve the lyophilized DNase I (1500
units) in 550 μl of the RNase-Free ddH2O. Mix gently by inverting. Divide it
into single-use aliquots, and store at -30~-15°C for up to 9 months.
Note: Thawed aliquots could be stored at 2-8°C for up to 6 weeks. Do not
refreeze the aliquots after thawing.
- Follow the procedure of RNA purification step 1-4.
- Add 350 μl Buffer RW1 to the RNase-Free Spin Column CR3. Close the
lid gently, and centrifuge for 30-60 sec at 12,000 rpm (~13,400 × g).
Discard the flow-through. - Preparation of DNase I working solution: Add 10 μl DNase I stock
solution (see Preparation of DNase I stock solution) to 70 μl Buffer RDD.
Mix by gently inverting the tube. - Add 80 μl DNase I working solution directly to the RNase-Free Spin
Column CR3, and place on the bench top for 15 min. - Add 350 μl Buffer RW1 to the RNase-Free Spin Column CR3. Close the
lid gently, and centrifuge for 30-60 sec at 12,000 rpm (~13,400 × g).
Discard the flow-through. - Follow the procedures of RNA purification step 6-9.
Total protein precipitation
- Add 4 volume of cool acetone (not supplied) or Buffer PR to the filtrate
from step 4 in RNA purification. Mix thoroughly and put on ice or at
-20°C for 10-30 min (the tube is not supplied).
Note: The protein precipitate obtained using acetone is difficult to
dissolve but the protein is higher in content than that using Buffer
PR. Please choose the appropriate solution according to specific
experiment. - Centrifuge for 10 min at 4°C at 12,000 rpm (~13,400 × g). Discard the supernatant.
- Add 100 μl of 95% cool ethanol to the protein pellet. Centrifuge at full
speed for 1 min, and remove the supernatant using pipet as much as possible. - Dry the protein pellet at room temperature.
Note: Incomplete drying may cause problems when loading the
protein onto a gel due to residual ethanol. Excessive drying makes
protein difficult to dissolve. - Add 100 μl or appropriate volume of Buffer SP and mix vigorously to
dissolve the protein pellet. The volume of Buffer SP used depends on
the amount of starting material and the downstream experiment.
Note: Protein obtained from Buffer SP could be used in SDS-PAGE and
Western blot, but not in Bradford protein assay. If the protein needs
to be quantified by Bradford protein assay, 5% SDS should be used to
dissolve the protein, or select a buffer compatible with the intended
downstream application.
SDS-PAGE procedure
- Add protein loading buffer to the sample and incubate for 5-10 min at
95°C to completely dissolve and denature protein. Then cool the sample
to room temperature. - Centrifuge for 1 min at full speed to pellet any residual insoluble
material. Use the supernatant in downstream applications such as SDSPAGE and western blot. The dissolved protein can be stored at -20°C for
several months or at 4°C for several days.
DP423-DNA/RNA/Protein Isolation Kit
All trademarks or registered trademarks appearing on this website are the property of their respective owners.
This product is for scientific research use only. Do not use in medicine, clinical treatment, food or cosmetics.
Need more info ? Contact us anytime. We’re here: Go2biotech
E-mail: maggie@go2biotech.com / morgan@go2biotech.com
Telephone:+86 755 8399 5017


